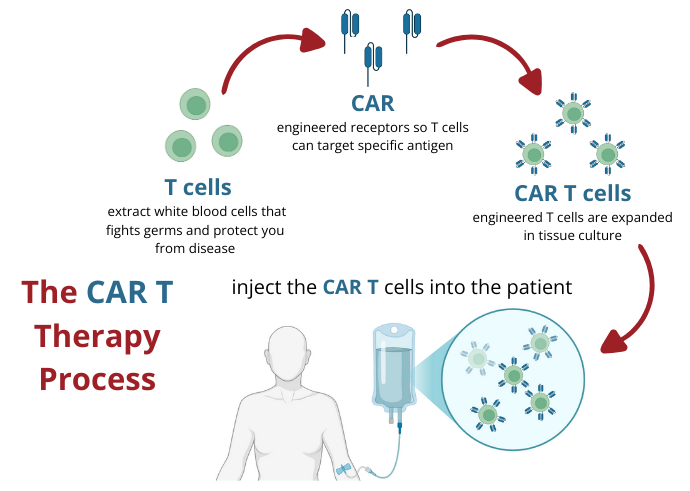
Spoke 10 – Focusing on CAR-T Therapies, Oncohematology, and Beyond
Spoke 10 sets its sights on gene therapies and beyond. This includes well-known CAR-T therapies, a strategy applied to numerous neoplastic diseases (such as lymphoproliferative diseases of the B cell line, leukemia, and lymphomas) and multiple myeloma, autoimmune, and hereditary diseases. Prof Franco Locatelli, President of the Higher Health Council, Professor of the Catholic University of the Sacred Heart, and Director of the Department of Hematology and Pediatric Oncology of the IRCCS Bambino Gesù Children’s Hospital in Rome coordinates the activities of Spoke 10. Here, researchers deal with preclinical development, Good Manufacturing Practices (GMP), and clinical trials of Gene Therapy Medicinal Products (GTMP).
Prof Locatelli shares, “We are the first on the European continent to conduct clinical trials for acute lymphoblastic T leukemia, which until recently did not seem susceptible to CAR-T cell therapies. These tumor cells do not have a specific target to exploit as a target for the CAR-T cells, so researchers use one that is also present in normal lymphocytes, thus triggering a fratricide, a term used to describe when CAR-T cells (derived from genetically engineered T lymphocytes) destroy each other. With a sort of molecular stratagem, we now can block the expression of the target molecule to ensure that it is not expressed on the surface of the CAR-T cells, thus preventing them from killing each other. Last September, in Nature Medicine, we published the first study entitled Fratricide-resistant CD7-CAR T cells in T-ALL, documenting the efficacy of CAR-T cells in this form of leukemia. This work has opened a study that, once phase one is completed, will be extended to three other European pediatric centers.”